
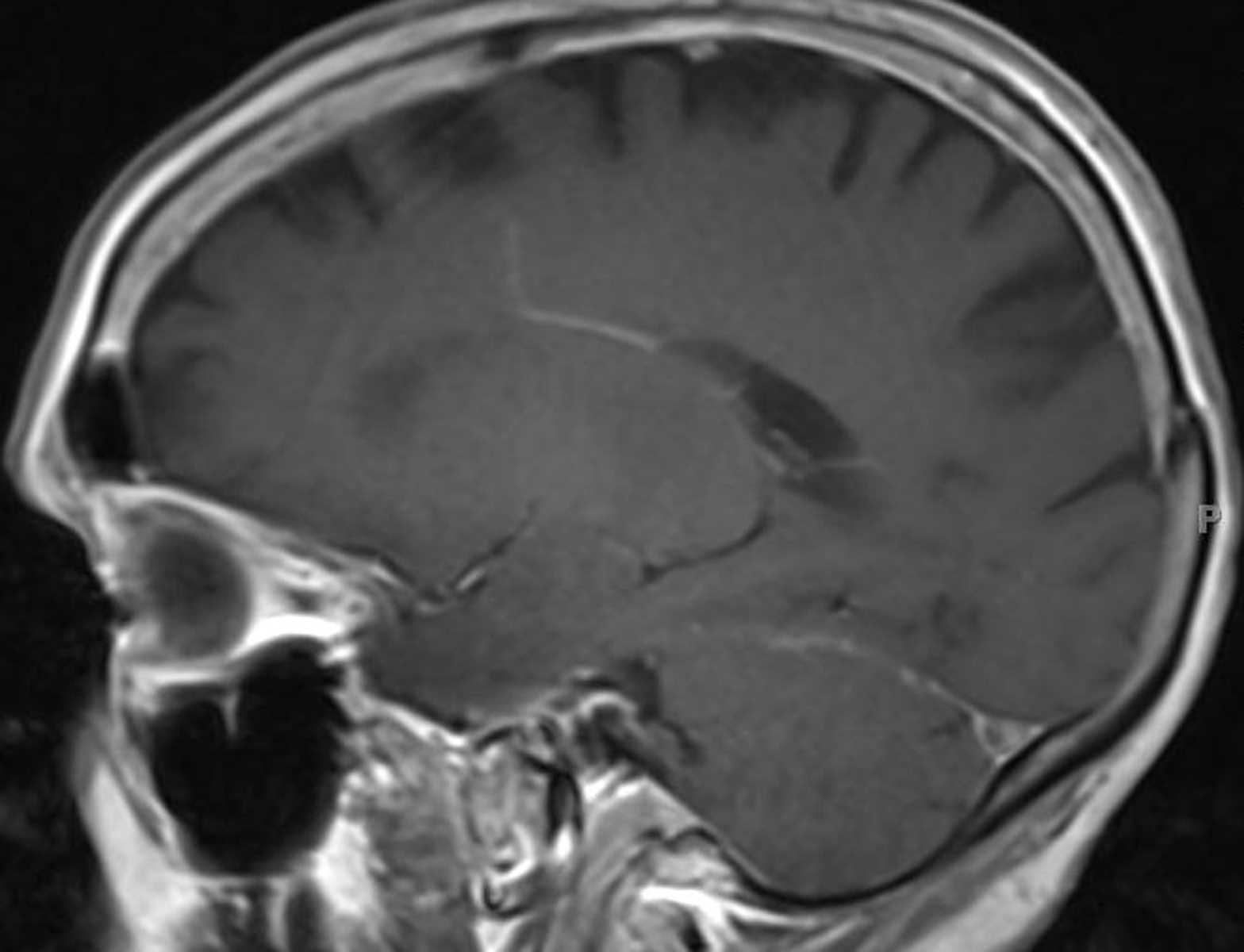
The third family has 2 different pathogenic mutations in MSH6 (c.3984_3987dupGTCA//c.3959_3962delCAAG), with 3 affected members who are compound heterozygotes.Īll children had surveillance brain MR imaging during the past 10 years. The second family has a MSH6 mutation, with 2 affected homozygous members. The first family has a PMS2 mutation (c.2458dupA), with 5 affected homozygous members. We performed detailed clinical and imaging analysis of all children from 3 different families that are carriers of DNA mismatch repair genes, each family with a distinct type of mutation. Our aim was to assess the prevalence of DVAs in children with CMMRD and to describe their phenotype to elucidate possible distinct features associated with CMMRD. 7 There is a known association between DVAs and cavernomas. However, DVAs have been documented as a rare cause of cerebrovascular bleeding and ischemic events. 8, 9 Most DVAs are asymptomatic and are found incidentally. Two or more DVAs coexisting in separate regions of the brain were observed in 7%–16% of described patients with DVAs. 7 The collecting vein crosses a variable length of brain parenchyma to join either the superficial or deep venous system.
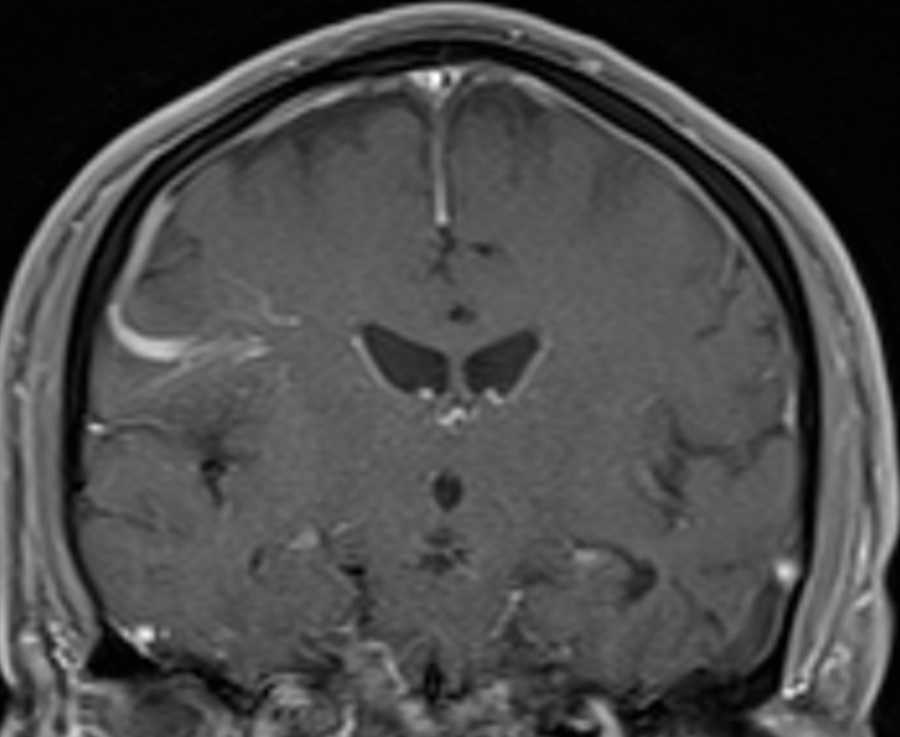
5, 6 A DVA is characterized by a cluster of venous radicles that converge into a collecting vein, resulting in the typical caput medusa appearance of the DVA. In Tel Aviv Sourasky medical center, we noted prominent developmental venous anomalies (DVAs) on routine brain MR imaging performed as a part of our surveillance protocol in patients with CMMRD.ĭVA is the most frequently encountered cerebral vascular malformation, with an incidence of 2.6%–6.4% in different studies. 1 In the suggested score, several features of the syndrome are assigned points a patient with a 3-point score or above is referred for genetic counseling. To facilitate CMMRD testing in patients with cancer, the European Consortium Care for CMMR-D suggested a score based on diagnostic criteria. 2, 4 It is important to arrive at a definite diagnosis of CMMRD in a pediatric or young adult patient with cancer as early as possible to allow the recommended surveillance for the patient and genetic counseling for family members. MR imaging of the brain, every 6 months, starting at diagnosis or birth, is part of the surveillance protocol recommended for these children by the European Consortium Care for CMMR-D 3 and the International Biallelic Mismatch Repair Deficiency Consortium. 1īecause CMMRD is a cancer-predisposition syndrome with a very high penetrance and most reported individuals are affected during childhood, surveillance protocols were developed aiming at early detection and interventions of these cancers.
#VENOUS ANOMALY SKIN#
1, 2 Nonmalignant manifestations of the syndrome include benign tumors such as adenomas and neurofibromas, features of neurofibromatosis type 1, predominantly café au lait macules, other hypo- and hyperpigmented skin alterations, pilomatricomas, and other features that were included in the suggested diagnostic criteria by the European Consortium. The most common malignancies are brain tumors (predominantly malignant gliomas, though other tumors are reported), lymphoid malignancies (most commonly non-Hodgkins lymphomas and leukemia), and gastrointestinal cancers (Lynch syndrome–associated tumors, especially colorectal cancer). ABBREVIATIONS: CMMRD constitutional mismatch repair deficiency syndrome DVA developmental venous anomalyĬonstitutional mismatch repair deficiency syndrome(CMMRD) is a cancer-predisposition syndrome characterized mainly by a high risk for developing cancer in childhood and young adulthood as well as nonmalignant features.


 0 kommentar(er)
0 kommentar(er)
